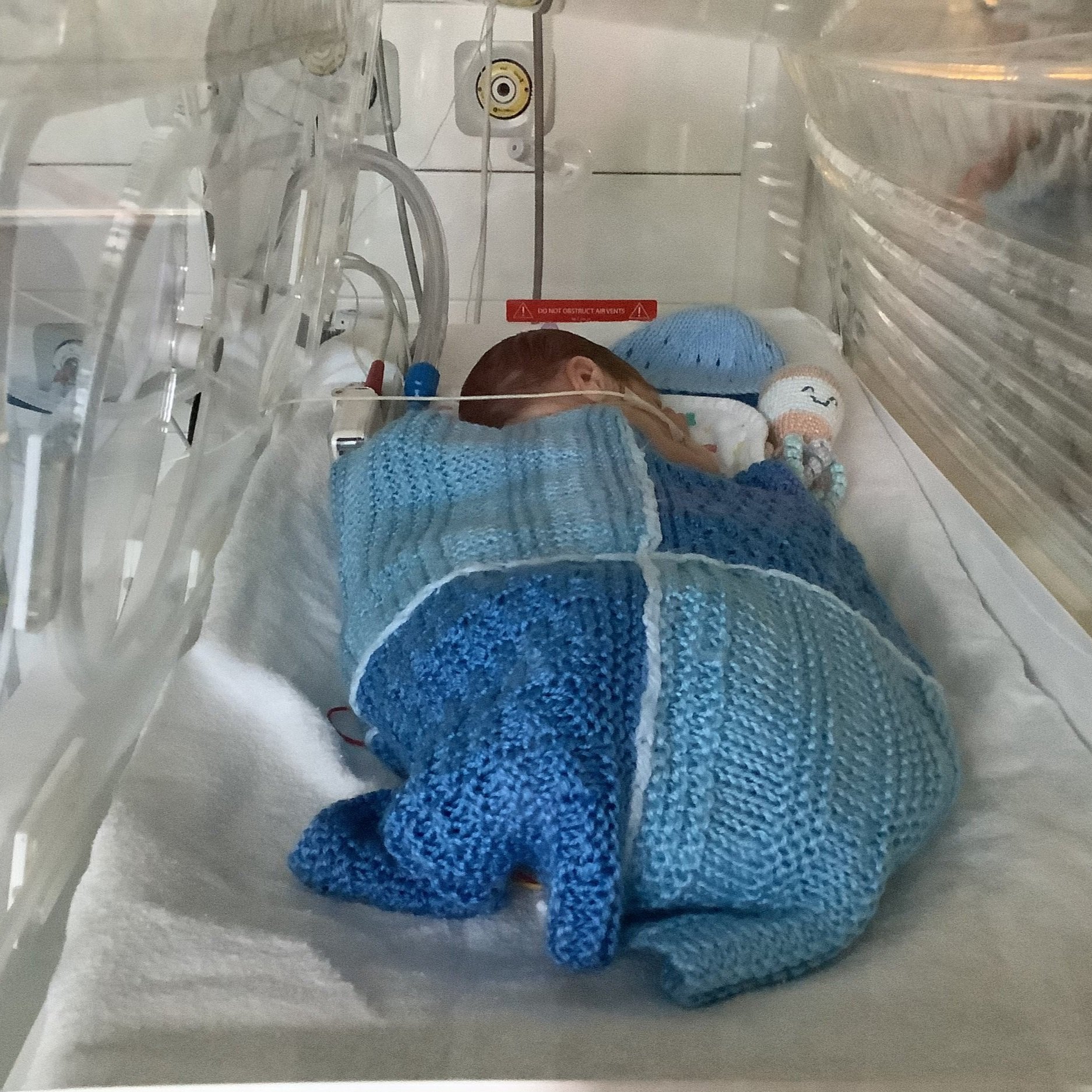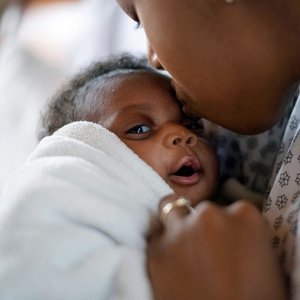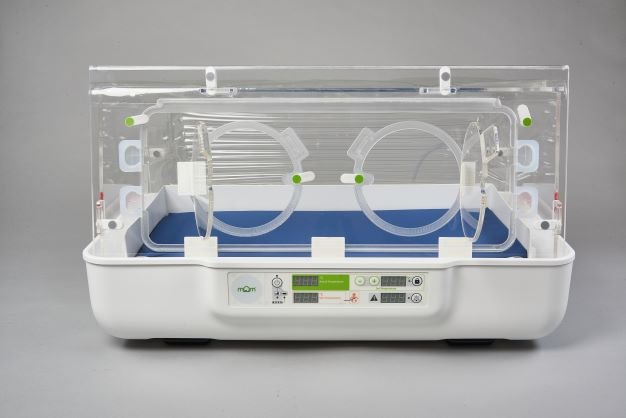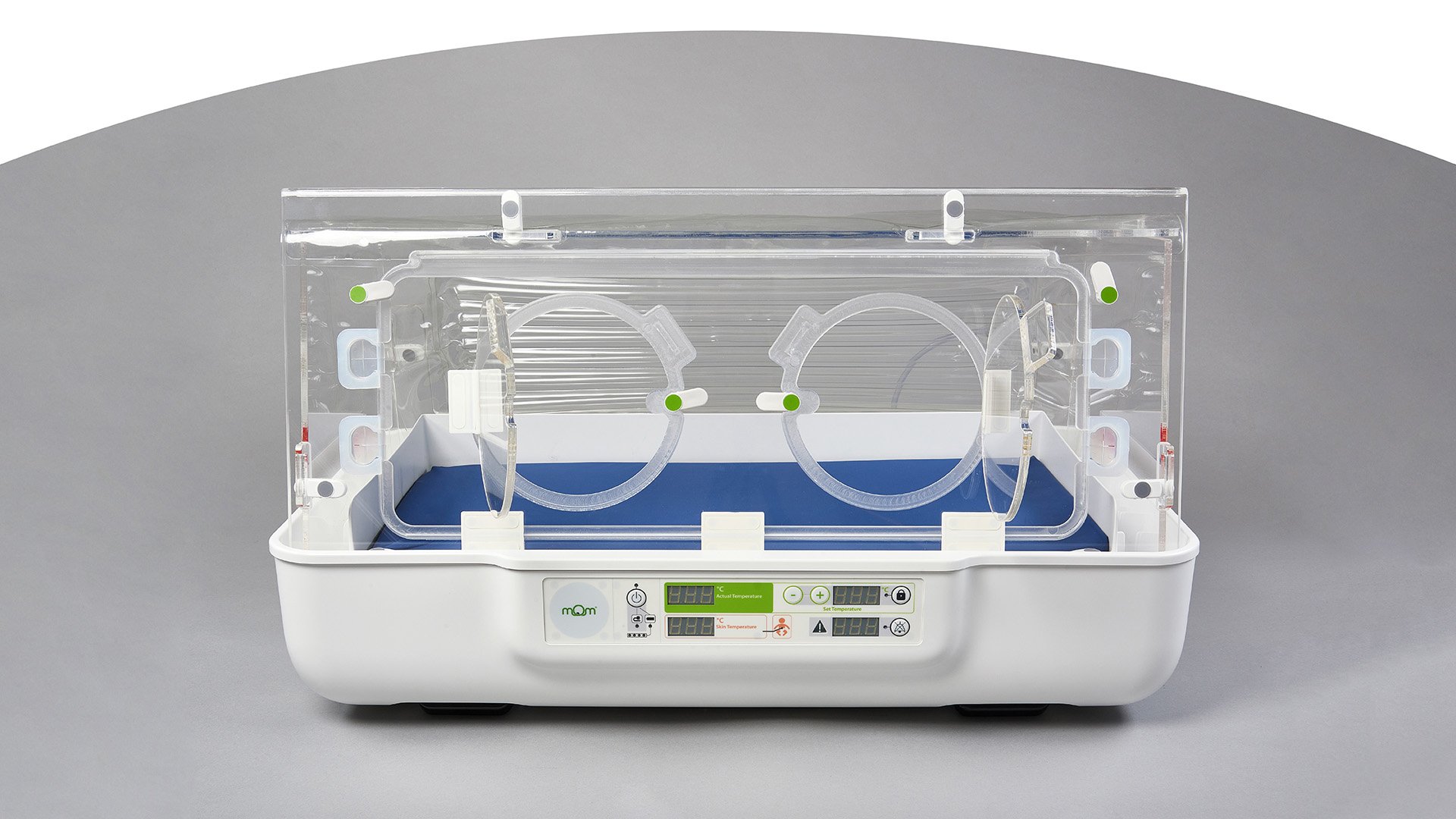
Keeping baby close and keeping baby warm is at the core of what the mOm Essential Incubator is all about.
Keeping baby close; compact, and lightweight with a small footprint, baby can be kept closer to parents allowing for those important bonds to be created.
Keeping baby warm; reducing the risk of hypothermia and the cascade of associated complications, helping reduce the need for short term admission to neonatal care.
Portable, easy to use with intelligent power and no servicing requirements it can be used across the care pathway from the birthing room to post-natal and neonatal care no matter where the clinical setting is located.
Thanks to St Peters NICU and the parents for allowing mOm to use these photos.
What We Do
mOm is on a mission to rethink how healthcare is delivered and accessed by solving problems from the ground up.
Our Purpose
We deliver simple, effective, and reliable technology solutions that can operate anywhere in clinical settings from remote & rural birth, intrahospital transport, and transitional care through to neonatal units across the spectrum of designation and capability.
Our Solution
Our flagship product, the mOm Essential Incubator, is a portable, compact, and lightweight infant incubator offering a safe thermoregulated environment for newborns to thrive in.
“By bravely challenging convention, [mOm] has created something that could save thousands of lives.”

mOm is supported by: